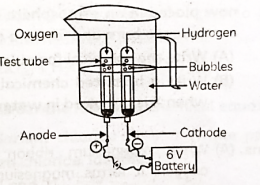
(A) Electrolytic decomposition of water/electrolysis of water. (B) The gas collected at cathode is hydrogen which is double the volume of oxygen collected at anode.
sample paper for class 10 science board exam 2026
CBSE Sample Paper Class 10 Science 2025-26
ANSWER:
(A) Electrolytic decomposition of water/electrolysis of water.
(B) The gas collected at cathode is hydrogen which is double the volume of oxygen collected at anode.
(C) 2H2O (l) + Electric current → 2H2 (g) + O2 (g) When an electric current is passed through water, it undergoes electrolysis. Two molecules of liquid water (2H₂O) decompose to form hydrogen gas (2H₂) and oxygen gas (O₂). Hydrogen collects at the cathode, while oxygen collects at the anode.
(D) Water is not a good conductor of electricity sulphuric acid is added in the water to make, it a good conductor of electricity.