Case A: Significant corrosion will be observed on the metal surface because the protective zinc coating on the metal readily react with oxygen and the moisture present in the moist air.
class 10 free sample paper for 2026 board exam
class 10 science half yearly 2025
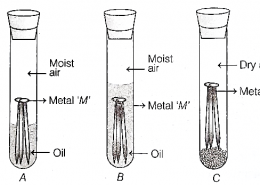
Case A: Significant corrosion will be observed on the metal surface because the protective zinc coating on the metal readily react with oxygen and the moisture present in the moist air.
Case B: No corrosion will be observed due to the oil layer with reacts with acts as a barrier and effectively inhibiting corrosion.
Case C: Minimal to no corrosion will be observed due to lack of moisture in the presence of dry air.