(a) Aluminium reacts with dilute HCl in an exothermic reaction, releasing heat and hydrogen gas, causing the temperature to rise.
(b) Sodium reacts vigorously with HCl due to its high reactivity, producing hydrogen gas rapidly with heat.
(c) Lead reacts slowly, releasing hydrogen gradually and forming few bubbles because it is less reactive.
class 10 science half yearly paper
class 10 science half yearly paper on tiwari academy
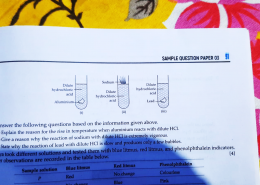
ANSWER: (a) The temperature rises because aluminium reaction with dilute HCl in an exothermic reaction, releasing heat along with hydrogen gas.
(b) Sodium reacts extremely vigorously with HCl because it is highly reactive, producing hydrogen gas rapidly with heat that can ignite the gas and causing a vigorous reaction.
(c) Lead reacts slowly with dilute HCl because it is less reactive, so hydrogen gas is released gradually, forming only a few bubbles.