Two observations that indicate a chemical change are: (1) Formation of a new substance, such as a gas, precipitate, or color change. (2) Release or absorption of energy, like heat, light, or sound, during the reaction.
2025-26 sample paper question available Satyam Tiwari Academy
extra cbse sample paper download on Tiwari Academy
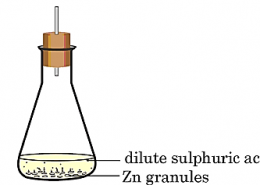
ANSWER: Observations to justify chemical change:
Hence, gas evolution and formation of a new product confirm that a chemical change has taken place.