Lime water turns milky because carbon dioxide gas reacts with calcium hydroxide solution to form calcium carbonate. When ethanoic acid reacts with sodium hydrogen carbonate, the products formed are sodium ethanoate (X), water, and carbon dioxide gas (Y).
cbse class 10 science half yearly sample papers
sample paper for class 10 science board exam 2026
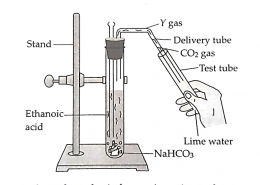
(a) Lime water turns milky because carbon dioxide gas reacts with calcium hydroxide solution to form calcium carbonate. Calcium carbonate is insoluble in water and appears as a white precipitate, giving the solution a milky appearance. This reaction is a common laboratory test for the presence of carbon dioxide gas.
(b) When ethanoic acid reacts with sodium hydrogen carbonate, the products formed are sodium ethanoate (X), water, and carbon dioxide gas (Y). The reaction demonstrates the typical acid–carbonate reaction, producing effervescence due to carbon dioxide release. The sodium salt formed is soluble, while the gas evolved can turn lime water milky.
(c) Carbon dioxide released from the reaction is confirmed using lime water. It reacts with calcium hydroxide to form insoluble calcium carbonate, which makes the solution appear milky. The balanced reaction is:
Ca(OH)₂ + CO₂ → CaCO₃ + H₂O. This simple test is widely used to detect carbon dioxide gas.