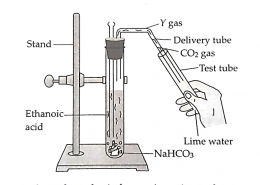
Lime water turns milky because carbon dioxide gas reacts with calcium hydroxide solution to form calcium carbonate. When ethanoic acid reacts with sodium hydrogen carbonate, the products formed are sodium ethanoate (X), water, and carbon dioxide gas (Y). cbse class 10 ...