The bond formed between X and Y is a covalent bond, because element X (carbon) shares its valence electrons with element Y (hydrogen – like or sodium) to achieve a stable configuration.
Download cbse sample paper for class 10 science
class 10 free sample paper for 2026 board exam
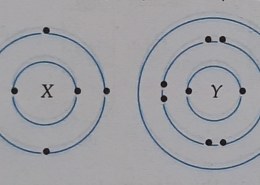
(a) The bond formed between X and Y is a covalent bond, because element X (carbon) shares its valence electrons with element Y (hydrogen – like or sodium) to achieve a stable configuration.
(b) Four valence electrons from X and one valence electron from Y participate in bond formation, as X needs to share electrons to complete its octet.
(c) The chemical formula of the compound is , as X forms four single bonds with four Y atoms.
(d) The compound is generally a poor conductor of electricity because covalent compounds do not have free ions or electrons to conduct current.
(e) When element Y (like Na) reacts with ethanoic acid , hydrogen gas is evolved, and the salt sodium ethanoate is formed.
CH3COOH + Na → CH3COONa + H2 ↑