X: Alcohol (ethanol, C₂H₅OH) – a good solventY: Hydrogen gas (H₂)Z: Sodium ethoxide (C₂H₅ONa) – used in making vegetable ghee Reaction: 2C₂H₅OH + 2Na → 2C₂H₅ONa + H₂↑ 10 years solved cbse sample paper for class 10 science 2026 board exam ...
Discussion Forum Latest Questions
Both A and B are true but R is not the correct explanation of A. Baking soda is used in Soda acid fire extinguishers because it release is carbon dioxide gas upon reaction with H2SO4. CO2 helps put out fire ...
The correct option is [C]. Sodium carbonate decahydrate (Na₂CO₃·10H₂O), also called washing soda, is used to remove permanent hardness of water. It reacts with calcium and magnesium salts, forming insoluble carbonates, softening the water effectively. Download cbse sample paper for class ...
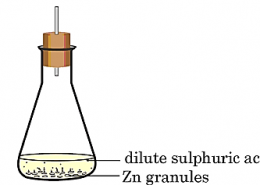
The correct option is [C]. When zinc granules react with sulphuric acid (X), zinc sulphate (ZnSO₄) forms and hydrogen gas (Y) is released. The hydrogen gas burns with a pop sound near a candle, confirming its identity. Tiwari Academy provides CBSE ...